Abstract
Introduction Both CD20 and CD19 are selectively and strongly expressed on the surface of diffuse large B-cell lymphoma (DLBCL) cells. For newly diagnosed and untreated DLBCL, the anti-CD20-targeted R-CHOP regimen is the standard of care. Tafasitamab, a humanized, Fc-modified, anti-CD19 monoclonal antibody, in combination with lenalidomide (LEN), has received regulatory approvals for the treatment of adult patients with R/R DLBCL not otherwise specified, including DLBCL arising from low-grade lymphoma, and who are ineligible for ASCT. A treatment strategy targeting both of these B cell surface molecules, and supplemented by LEN to enhance the cytotoxicity activity of tafasitamab and rituximab, may limit target evasion and reduce resistance to R-CHOP.
First-MIND (NCT04134936) is a Phase Ib randomized study to assess the safety and tolerability of R-CHOP + tafasitamab ± LEN in patients with previously untreated and newly diagnosed DLBCL, and an International Prognostic Index (IPI) score of 2-5. The primary analysis demonstrated the feasibility of adding tafasitamab + LEN to R-CHOP without impairing its dosing and scheduling, with toxicities similar to those expected with R-CHOP alone (ASH 2021; #3556). The combination of R-CHOP and tafasitamab + LEN as first-line therapy is being investigated further in the global, randomized, Phase III frontMIND study (NCT04824092) in untreated patients with DLBCL and an IPI score of 3-5.
Here, we report the 18-month follow-up analysis from the First-MIND study in all patients and in patients with an IPI score of 3-5.
Methods Eligible patients were randomized 1:1 to six 21-day (D) cycles of either R-CHOP (R-CHOP, D1-5) + tafasitamab (12 mg/kg IV, D1, 8, 15) (Arm T) or R-CHOP + tafasitamab + LEN (25 mg orally, D1-10) (Arm T/L). The primary endpoint was incidence of treatment-emergent adverse events (TEAEs). Secondary endpoints included overall response rate (ORR) and PET-negative complete response (CR) rate at end of treatment (EoT). Safety was assessed using the NCI CTCAE V5.0, and tumor measurements by PET/CT or PET/MRI at EoT were performed according to Lugano 2014 criteria. Minimal residual disease (MRD) was assessed using immunoglobulin gene next-generation sequencing in cell-free DNA.
Results At the data cut-off (May 5, 2022), of the 66 patients randomized (Arm T, n=33; Arm T/L, n=33), a total of 27 patients (40.9%) were still on study (Arm T, n=13; Arm T/L, n=14) with a median follow-up of 17.6 months for progression-free survival (PFS). Baseline characteristics were well balanced: 60.6% of patients in Arm T and 66.7% in Arm T/L had an IPI score of 3-5; 94.0% and 87.9% had an ECOG PS of 0-1, respectively; and 93.9% of patients in both Arms were Ann Arbor stage III/IV.
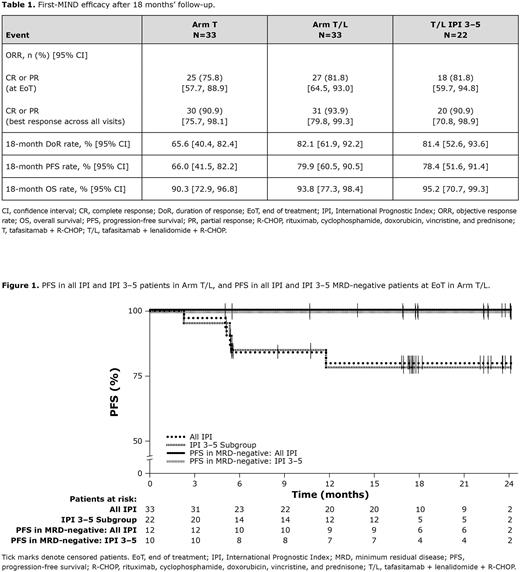
ORR at EoT visit and best response across all visits were higher in Arm T/L, as were 18-month DoR, PFS, and OS rates (Table 1). In patients treated in Arm T/L and with an IPI score of 3-5 (n=22), ORR and 18-month DoR, PFS, and OS rates were comparable with the overall Arm T/L cohort (Table 1). The 12-month PFS rate by MRD status at EoT in Arm T/L was 100% in MRD-negative patients (n=12) (Figure 1) and 67% in MRD-positive patients (n=3). MRD-negativity at Cycle 2 Day 1 appeared to be predictive for durable PFS responses.
The frequency of Grade ≥3 TEAEs was 72.7% in Arm T and 90.9% in Arm T/L; the most common TEAEs were neutropenia, anemia, leukopenia, and thrombocytopenia in both arms. Serious TEAEs occurred in 42.4% in Arm T and 51.5% in Arm T/L, with no difference between treatment arms in the incidence of febrile neutropenia (15.2%), and an incidence of infections and infestations of 9.1% in Arm T and 6.1% in Arm T/L.
Conclusion Adding tafasitamab in combination with LEN to R-CHOP shows numerically higher clinical efficacy than adding tafasitamab alone, and is consistent with the synergy between tafasitamab and LEN, leading to durable responses in treatment-naïve patients with DLBCL. The long-term safety profile of tafasitamab ± LEN when added to R-CHOP showed no new safety signals to those reported previously. Although the sample size is limited, patients with an IPI score of 3-5 treated with tafasitamab + LEN + R-CHOP showed efficacy comparable to that of the overall treatment arm cohort, including MRD-negative patients who remained disease-free for ≥18 months. frontMIND will further evaluate tafasitamab + LEN + R-CHOP in previously untreated patients with high-intermediate and high-risk (IPI score 3-5) DLBCL.
Funding MorphoSys AG
Disclosures
Nowakowski:Bantam Pharmaceutical: Consultancy; Blueprint Medicines Corporation: Consultancy; Celgene Corporation/Bristol Myers Squibb: Consultancy, Research Funding; Curis, Inc.: Consultancy; Daiichi Sankyo Inc: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Genentech, Inc: Consultancy, Research Funding; Incyte: Consultancy; Karyopharm: Consultancy; Kite Pharma Inc.: Consultancy; Kymera Therapeutics: Consultancy; MorphoSys US Inc: Consultancy; NanoString: Research Funding; Ryvu Therapeutics: Consultancy; Selvita: Consultancy; TG Therapeutics: Consultancy; Zai Lab: Consultancy. Duell:Regeneron: Research Funding; Incyte: Consultancy; MorphoSys: Consultancy, Research Funding. Kopeckova:Laboratoires Pierre Fabre: Current equity holder in private company; Novartis: Current equity holder in publicly-traded company, Honoraria; Viatris: Current equity holder in publicly-traded company; EISAI: Research Funding. Trneny:Zentiva: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Morphosys: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Hoffman-La Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding. Burke:Kymera: Consultancy; Morphosys: Consultancy; Nurix: Consultancy; Kura: Consultancy; Epizyme: Consultancy; Bristol Myers Squibbs: Consultancy; BeiGene: Consultancy, Speakers Bureau; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy; Abbvie: Consultancy; Roche/Genentech: Consultancy; SeaGen: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy; Verastem: Consultancy; X4 Pharmaceuticals: Consultancy. Waldron-Lynch:MorphoSys, US Inc., Novartis AG (immediate family member): Current Employment, Current equity holder in private company. Wagner:MorphoSys: Current Employment, Current holder of stock options in a privately-held company, Other: Travel and accomodation expenses. Mukhopadhyay:MorphoSys, AG: Current Employment. Blair:BMS: Current equity holder in private company; MorphoSys, AG. Inc: Current Employment. Belada:Gilead Sciences, Roche, Takeda: Other: travel expenses; Gilead Sciences, Janssen-Cilag, Roche, Takeda, MorphoSys AG, Debiopharm Group: Consultancy; Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys AG, Pharmacyclics, Archiden Biotech, Reddy: Research Funding.
OffLabel Disclosure:
Tafasitamab is a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody. In combination with lenalidomide (LEN), it received accelerated approval in July 2020 for adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), including arising from lowâ€'grade lymphoma, who are ineligible for autologous stem cell transplant (ASCT). Following FDA approval, we are now evaluating the safety and efficacy of tafasitamab in combination with LEN as an add-on to first-line therapy with R-CHOP in newly diagnosed patients with DLBCL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal